Synthesis and Dispersion of Ni-Doped Cu2ZnSnS4
Camellia Panatarani, Hera Redianti, Ferry Faizal, Eka Cahya Prima, Brian Yuliarto, I Made Joni
Key Engineering Materials 860, 42-50, 2020
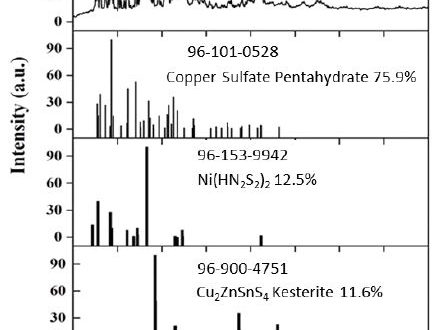
This paper reports the preliminary study on the synthesis of Ni doped CZTS (Cu2ZnSnS4:Ni) particle 5 at.% of Cu by solution method and dispersion of the obtained particles by beads mill method at various dispersing agents (SDS, CTAB, and Tween80). The phase transformation of the obtained particles was analyzed from the XRD spectra and XRF elemental analysis. The phase transformation and amount of Ni-doped to particles was predicted employing commercially available analytical software tool Match! Version 2.x. Moreover, the dispersion characteristics were investigated includes size, size distribution, and zeta potential of bare particles in comparison to various dispersing agents. This characteristic related to the future application of CZTS as an absorber in a thin-film based PV. The XRD analysis showed that the obtained particle contained crystal structure of copper sulfate pentahydrate (75.9 %), Ni(HN2S2)2 (12.5 %), and Cu2ZnSnS4 (11.6%). The XRF elemental analysis showed that amount of Ni-doped was 6.8 at.%; it was higher than the initial design amount of Ni doping. The dispersion of suspended particles was the majority (90%) with an average size of 3.06 µm and only 10 % with size 255 nm. Beads-milling of particles without dispersing agents did not disintegrate agglomerated particles. It is highlighted dispersion only using magnetic stirred with SDS dispersing agent provides the best suspension with a majority (60%) in 166 nm and only 30 % with average size 3.06 µm with relatively high zeta potential (-17 mV). It was concluded that the presence of a multi-phase crystal needs to be resolved either by proper calcination at a higher temperature than 400°C or further heating at a higher temperature during film preparation. High-energy centrifugation of zirconia beads mill caused desorption of adsorbed steric stabilization of dispersing agent under investigation. Further investigation on the coating process to facilitated laboratory fabrication of thin-film absorber with SDS as a dispersing agent is necessary to carry out concerning the properties of the thin-film.
The paper can be assessed at the link https://www.scientific.net/KEM.860.42